|
Robots and autonomy: The EcoBot project |
|
For a robot to behave truly autonomously it will need not only to use its energy in an effective way but also extract this energy from its environment. This requires the robot to convert energy from natural raw materials and also deal with replenishing reserves and waste management. A major barrier to the widespread deployment of autonomous robots in remote areas (away from power utilities) is the availability of energy. The present work represents a first step towards addressing this fundamental issue. Industrial applications include those requiring ‘release and forget’ robots; where robots are required to accomplish a mission usually in dangerous or undesirable for people areas (such as perimeter/pollution/predator monitoring) with minimum maintenance. The waste disposal industry will be interested in extracting energy from food waste. This new technology also offers the prospect of ‘gut sensors’ for environmental sampling. Toxins/pollutants of interest could be monitored by its effect on microbial efficiency. Long term, this new technology could be integrated with EAP/active polymers to build self-powered actuators. In many ways the system will mimic real digestion
(e.g. in the use of micro-organisms within a tubular membrane to break down the
food components and produce reducing power) and respiration (e.g. using air to
provide oxygen to an electrochemical half-cell to create useful energy). We
believe that this robot will be the first robot capable of autonomous behaviour
powered by an on-board Microbial Fuel Cell, i.e. that will employ artificial
metabolism. This system is CO2 neutral; it does not utilise fossil
fuels and involves no net CO2 production other than that which occurs
naturally in vegetable decomposition. |
|
The EcoBot project focuses upon the creation of a smart on-board artificial digestion system for an autonomous robot. The artificial ‘gut’ is currently designed around novel Microbial Fuel Cell technology (MFC). Energy output from the MFC is not, at this stage, intended to sustain continuous motion activity (although computation can be continuous). The robot will therefore employ a ‘pulsed’ behaviour mode. The goals of this study are to (1) produce new types of MFC that in contrast to conventional MFC can operate over long periods of time (2) produce sufficient power for autonomous robot operation (3) utilise natural food substrates. Energy needs to be accumulated first before being accessible to the robot and we argue, is more realistic than simply managing a fixed energy budget. This issue will eventually need to be addressed by autonomous robots, but at the present moment, development of efficient on-board MFC’s is the main challenge. These need to be portable, replenishable and sustainable and capable of using a wide range of natural foodstuffs. By judicious choice of different food/flora combinations, the techniques envisaged will be capable of exploiting any organic food source on land and sea, and can potentially therefore be employed by terrestrial and marine robots. |
|
A picture of the analytical model of the MFC is shown below in Figure 1. |
|
Figure 1. Analytical type of MFC with dimensions h = 8 cm, w = 6.5 cm, b = 5 cm. (click the picture for a larger image) |
|
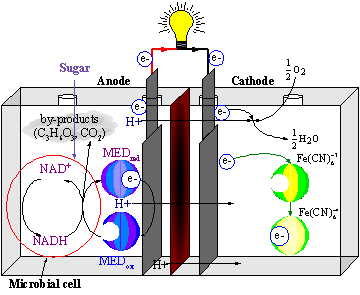
One type of MFCs, which we name generation I (Gen-I), produce power by
use of a microbial cell-permeable chemical mediator, which in the oxidised form
intercepts a proportion of NADH (nicotinamide adenine dinucleotide) within the
microbial cell and oxidises it to NAD+. The now reduced form of
mediator is also cell-permeable and diffuses away from the microbial cell to
the anode where, the reduced redox mediator is then electro-catalytically
re-oxidised (see Figure 4 below). In addition, cell metabolism produces protons
in the anodic chamber, which may migrate through a proton selective membrane to
the cathodic chamber. In the latter, they are consumed by ferricyanide (Fe3-(CN)6)
and incoming electrons (via the external circuit) reducing it to ferrocyanide
(Fe4-(CN)6 ). The oxidised mediator is then free to
repeat the cycle. This cycling continually drains off metabolic reducing power
from the microbial cells to give electrical power at the electrodes. Figure 2
shows a schematic representation of the redox reactions within an MFC is
shown in the figure below. The LHS represents the anodic chamber and the RHS
represents the cathodic chamber with electrodes shown in grey colour. In the
middle shown in dark brown is the proton permeable membrane. |
|
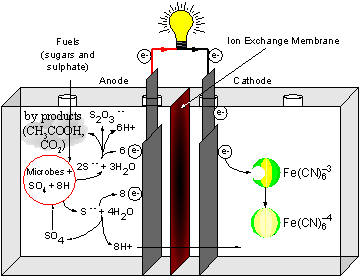
There are other ways of extracting energy from microbes in a MFC, apart from using synthetic mediators. For example, by using a different species of bacteria that can reduce sulphate to sulphide, the electron extraction can be performed naturally. Sulphate (oxidised) is taken up by the bacterium and reduced to sulphide. This then electro-actively diffuses outside the bacterium cell and re-oxidises at the electrode surface. The re-oxidised sulphate is then re-reduced by the bacterium, in a repetitive cycle. Sulphate can be found in polluted water, so these systems can be used to generate electricity fuelled with wastewater. We call this type of MFC a generation-II (Gen-II). A schematic diagram of the Gen-II MFC is shown in Figure 3. |
|
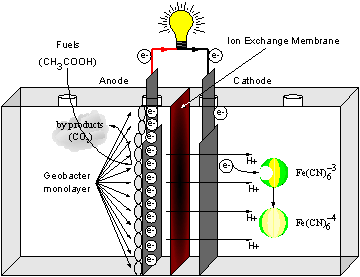
Furthermore, anodophillic microbes like Geobacter sulfurreducens or Rhodoferrax ferrireducens, when employed as biocatalysts, can form a biofilm on to electrode surface and use this as part of their respiration. This means that electrons are passed on to the anode electrode without the use of any mediators – synthetic or natural! G. sulfurreducens can utilise acetic acid (acetate) which is once again a constituent of polluted water. A schematic diagram of a Gen-III MFC is shown in Figure 4. |
|
Figure 4. Gen-III MFC |
|
Both of these types of MFC systems have been previously reported in the scientific literature by other workers (Habermann and Pommer, 1991, Lovley et al 2003) but we have also used them in our experiments. Experiments carried out with the sludge MFCs, indicate that the microbial flora found in sludge has similar properties to those of Gens II and III described above. Currently we are investigating the sludge MFCs to understand whether they are a combination of Gens II and III, either or none of the two. It is possible that sulphate reducing bacteria in the sludge microbial flora are using the sulphate which is present in the sewage water, in a similar manner as D. desulfuricans in Gen-II. This is yet to be proved, and therefore there may well be other intermediaries. On the other hand, biofilm may be formed on to the electrode surface, by anodophillic species in the microbial flora, which could be blocking the oxidation of any other electron carrying substance. EcoBot II is certainly not the first in the world to use bacteria
however. The first was Wilkinson’s Gastronome (Chew-chew) in 2000, which
employed MFCs to charge up a bank of Ni-Cd batteries. Power was generated by E.
coli fed with refined sugar, and a synthetic mediator (HNQ) enhanced the electron
transfer process.
As far as MFCs are concerned, we are not the first group in the world to exploit sludge, and certainly not the first in the world to use the O2 cathode. To the best of our knowledge the first sludge MFC reported in the scientific literature was from Habermann and Pommer back in 1991, in which case they had a stack of MFCs running continuously for 5 years. In later years, Park and Zeikus (2002) had done some significant experiments with sludge, E. coli and neutral red. And more recently, but certainly before us, Logan’s group at Penn Sate, have illustrated power generation from sewage sludge and more importantly with and without using a proton exchange membrane.
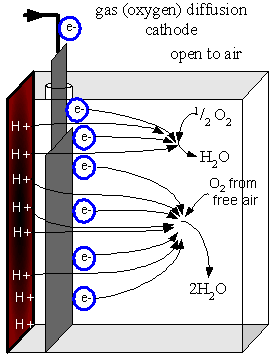
Shown below as a half-cell is a schematic diagram how the oxygen (O2)
diffusion cathode works. This can be used with any type of MFC, although O2
diffusion through the membrane can decrease the efficiency of an anaerobic
anode. |
|
Our research team, comprising Prof. |
| Questions on the IAS Laboratory to Chris Melhuish: chris.melhuish@uwe.ac.uk |